Careers in industrial science continue to expand with positions opening up in both government and private institutions, especially in the area of research and manufacturing. Graduates can choose from a range of careers in agricultural and biological sciences, the information and technology sector, food and pharmaceutical companies, as well as mining and mineral exploration.
With the unparalleled expansion of scientific knowledge, industrial scientists have the opportunity of working at the leading edge of scientific developments no matter whether they have a leaning towards biology, chemistry or physics.
There will be a career path in industrial science in a variety of fields, and this article looks at five fascinating careers to consider.
1. Industrial Microbiology
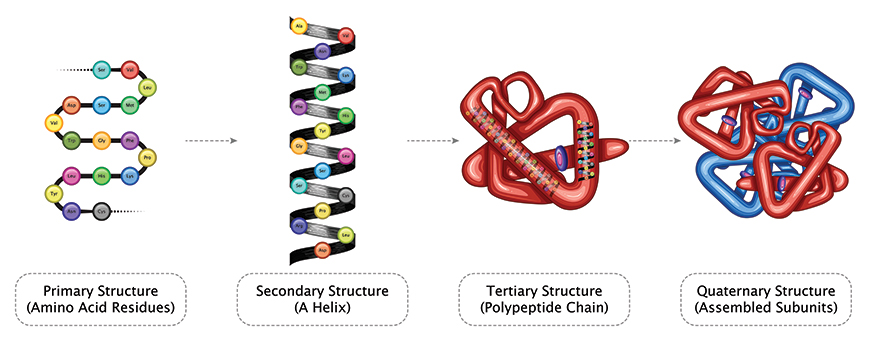
If you have a penchant to work in a multidisciplinary scientific environment, then industrial microbiology or biotechnology could interest you. Processes and production problems often take scientists in a variety of directions which means that an industrial microbiologist has to be adaptable across such fields as bioengineering, biochemistry and molecular biology. Career pathways can lead you into fields such as antibiotics and vaccines as well as many other healthcare products and even food and beverages which are produced by microbial activity, for instance, cheeses, yoghurts.
2. Environmental Engineering
Environmental engineering suits graduates who are concerned about the man-made environment and issues relating to water quality, waste disposal, air quality and dealing with contaminated land. Today, research into the prevention of pollution is supported by government and private agencies alike and graduates can expect to work with mechanisms of sustainability in either private companies or government research facilities.
3. Chemical Engineering
Chemical engineering provides a practical link between the theory of science and manufacturing. Industrial scientists with a preference for working in this area will be involved in designing of equipment and development of large chemical manufacturing processes in a variety of industries including photography and photographic equipment, manufacturing chemicals and health care products
4. Academic Research
Most academic careers in the area of industrial science will attract high achieving practitioners looking to develop their research and, naturally, to teach within universities. Professorial appointments are highly regarded and provide satisfying careers for experienced scientists. Although opportunities are limited, with the expansion in industrial scientific jobs as a whole, academic posts are becoming more frequently advertised.
5. Nanotechnology
Within the emerging realm of nanotechnology, jobs are being created across a diverse range of activities. From creating cosmetics and researching the nature of matter, to medical diagnostics and developing better batteries are just a few opportunities that provide blossoming careers for industrial scientists. It is safe to say there is a revolution in manufacturing and in production of new materials. The new ways in which these are made is largely under the direction of a highly qualified industrial scientist. You could find yourself working for a sports equipment company or the army. The choices are almost endless.
Industrial Science Growth
The outlook for employment in the area of industrial science is rapidly increasing. Government predictions of job growth show that this growth will continue for at least the next three years unabated. Even in times of slower employment growth, it is apparent that many companies will continue to research and develop new products requiring industrial science expertise.
Your future
Regardless of the field chosen, most people working in industrial science will gain first hand experience with cutting edge analytical measurement techniques. Measurement technologies such as Laser Diffraction, Dynamic Light Scattering, Spectroscopy, HPLC and Rheology are widely used in industrial science jobs. With the help of these cutting-edge technologies supplied by ATA Scientific, people around the world are expanding development of exciting new products that will shape our future world.



 02 9541 3500
02 9541 3500